News & Insights
News & Insights
BY BPD
For most health systems, the dream of the COVID-19 vaccine is becoming reality. It’s affecting hospital operations, finance, and especially clinical teams. Yet, it’s also affecting you, the marketers and communicators. Many of you will be focused in the coming weeks to firm up your vaccine communications plans, just as leadership works tirelessly to develop your hospital or health system’s corporate strategy on the matter.
We’re here with brand new original research to help you inform your plans in today’s consumer and internal audience truths.
Over the past month, Revive has re-fielded its consumer and physician & nurse polling research to give marketers and communicators an updated sense of how the vaccine is regarded among consumers, physicians, and nurses.
Note: we’ll cover some of the top findings here, but we’re more than happy to set up a briefing call with any health system marketing leader looking for guidance on how to proceed with effective marketing and communications related to the vaccine. Click here, and we’ll be in touch.
Here are the three new data points to help you ground your strategy in audience truths:
1. Consumer willingness to immediately receive the COVID-19 vaccine jumped up 10% over the past two months.
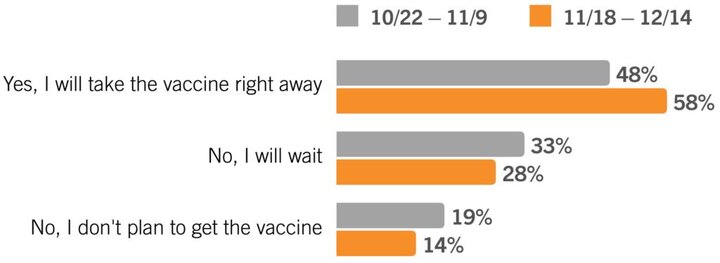
These updated findings are very encouraging, but they should also cause us to pause. Increased willingness to take the vaccine could be a good thing for public health, but an over-eagerness to get the vaccine could also prove problematic since it seems unlikely that the general public will have access to the vaccine anytime in the next couple of months or even beyond. Especially as timelines shift and availabilities change.
BOTTOM LINE: Be clear in your communications about who will get the vaccine on what timeline, so you can manage the expectations of your community.
2. Respondents cite safety concerns as the number one reason for delaying or forgoing the vaccine, but they can be convinced.
Forty-two percent of consumers want to delay or forgo the COVID-19 vaccine, and 65% of physicians and nurses indicated they did not want to be in the first wave.
Why? The leading reason is safety concerns. We already knew this from our past surveys, but the new revelation of our most recent poll was in an open-ended question we asked. For consumers, we asked, “How will you know that the COVID-19 vaccine is safe enough for you?” And for physicians and nurses, we asked, “How will you know that the COVID-19 vaccine is safe enough to order/prescribe for your patients?”
After combing through each qualitative response and categorizing them, the top-cited signs that the vaccine is “safe enough” are listed below:
I will know the vaccine is safe enough when: enough time passes to study the long-term effects.
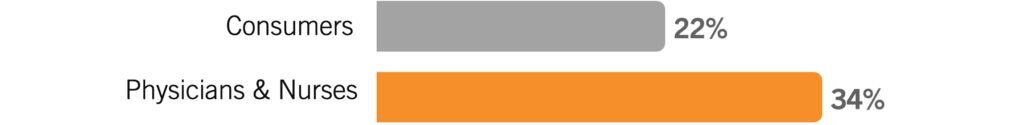
Respondents whose answers fell in this category typically focused on the concern that we haven’t been testing this vaccine long enough to know what side-effects may pop up months or even years down the line.
Example verbatim answers include:
- “It takes many years to confirm a vaccine’s safety profile.” – a primary care physician
- “I think the safety of the vaccine will only become clear in future generations.”– a nurse
- “I’ll get on board after 2-3 years of real-life side effects studies.” – a consumer
Why this matters: health system communicators should realize that they have a problem that is somewhat unsolvable. Physicians and nurses in your system, and even consumers in your community, may be hesitant about the vaccine’s safety profile for reasons we simply can’t refute at this stage. We must point to the data and the thoroughness of FDA and health system epidemiologist review of data as well.
I will know the vaccine is safe enough when: the people before me get the vaccine and end up safe.
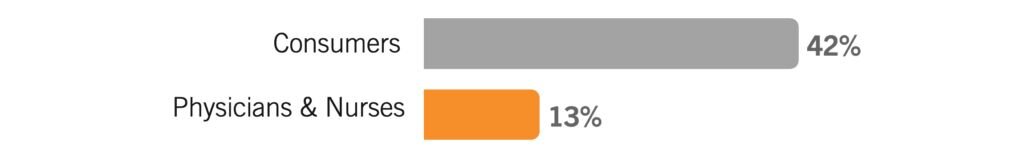
Consumers and physicians (but especially consumers) are watching to see how the vaccine affects those before them in line before they decide it’s safe enough for them. This is pure critical mass theory: once enough people have decided it’s safe enough for them, consumers are likely to jump on board. This answer category was less about concerns around long-term side-effects and more about “seeing it for themselves.”
Example verbatim answers include:
- “If the majority of the public takes it and turns out okay, then I’ll do it.” – a consumer
- “After others have taken it (in the healthcare field) and had no adverse side-effects” – a nurse
- “Once word starts spreading of the efficacy and safety in the real world.” – a primary care physician
Why this matters: As communicators, there’s a massive opportunity to elevate the stories of every day and influential people who choose to get the vaccine and experienced minimal or no side-effects. This could go a long way in helping consumers to warm up to the idea — especially if they are concerned about safety. Looking for ways to encourage front line caregivers to share their experience and build an echo chamber will be important.
BOTTOM LINE: Success stories will help curb safety concerns for internal and external audiences. Find opportunities to show real-world wins, and set checkpoints where you can elevate or publish favorable side-effect studies and reports at the 3 month, 6 month, and 12 month intervals.
3. One-third of consumers aren’t sure they’ll be on the same page as their doctors when it comes to whether/when to receive the COVID-19 vaccine.
Related Blog Posts
Stay in Touch
Stay in Touch
Receive our updates, industry news and insights.


